甲烷冰与全球暖化
大量的甲烷 (CH 4 ) 在地球表面冻结成冰状称为甲烷冰,即甲烷水合物 (methane hydrate) 。该水合物几乎可与任何气体化合,由水分子形成笼形 (cage) 结构,将气体团团围住, ( 「气水包合物 ( clathrate ) 」一词通常是描述含有气体的固体,被笼形结构所包围,若该结构主要由水分子构成,即称之为水合物 ( hydrate )) 火星上有二氧化碳,而地球充满甲烷水合物,而多数都沉积在海床底下,但有些就成为永冻层。
甲烷冰看起来似乎是很不稳定的物质,如果天气过于暖化,就会溶解并浮在水面上。甲烷是一种强烈的温室气体,它可以降解成二氧化碳,变成另一种温室气体,累积在大气中,就像燃烧石化燃料产生二氧化碳一样。而且其囤积量不容小覤,可能远比传统燃料生成量为多,可以想见,气候变化会影响此类二氧化碳的囤积量,但我们对灾难电影中甲烷冰的潜在危险性,了解有多少呢?
海洋水合物 -- 大部份的甲烷水合物沉淀在海里,因此被称为地层囤积。有机碳是来自上百万年前的浮游生物残骸,在数百公尺深的海床底下,细菌从死去的浮游生物制造出甲烷。假如甲烷的产生速度够快,有一些即被冻结成甲烷冰。有数十亿吨的甲烷,便以这种形式囤积在海里[资料来源: Buffett and Archer, 2004; Milkov, 2004 ]为对照起见,碳生成量最大的传统燃媒,其标准产碳量仅约 5000G 吨[资料来源 : Rogner, 1997 ]

有时甲烷在地底会移动并聚集在某些地方,形成所谓的结构性水合物囤积。譬如墨西哥湾,基本上是个有漏洞的油田[资料来源 : MacDonald et al., 2005 ]。 气体的游移与聚集,意味着浓缩度会提高,甚至会高到形成块状矿床的程度,变成纯块状的水合物。第二个后果是该些水合物,可以在离海底较近的地方、甚至在海床底下被找到。
气候过于暖化会使水合物溶解。在海平面 500 公尺以下 ( 在北极为 200 公尺 ) 的海水算够冷,在海床底下,温度会随深度增加而递增,沿地温梯度增加。若在某一深度的地底,因为太过暖化,而水合物未能埋在比原来更深的地底,就会溶解。在一个稳定的水合物区域下方,通常有一层气泡层。气泡所反射声波震动讯号,可以在全球的地震勘测清楚取得[资料来源 : Buffett, 2000 ]气泡层的丘陵与山谷,和海床的丘陵与山谷几乎平行,所以这一层被称为 海底仿拟反射 层 ( BSR ) 。

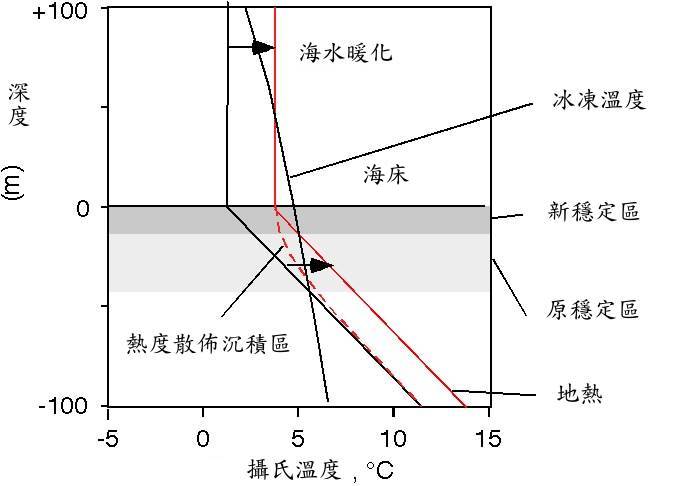
现在让我们为 沉积柱 上方的水加温。最后得到的地热新温度曲线图,其斜率几乎与之前的一致。水合物稳定区将因 沉积层 温度的升高而变得更薄。
值得密切注意的是,它是从底部变薄,而不是由顶端表面变薄。水合物的原稳定区基地部分,出现自发性的溶解。
假如稳定区仍然存在,则其在沉积柱内的范围,也要比在最新释放出的甲烷气泡中为浅,才能形成冷阱, 以防止甲烷气体散失。然而在地震研究上常发现 ” 无反射区 ( wipeout zones )” ,这是海底仿拟反射的死角,且在海底仿拟反射之外的沉积层,其所有层状结构都呈平滑状。该些区域被认为是气体已突破沉积结构,散发到海水里[资料来源: Wood et al., 2002 ]有一理论主张流体往上移动时会增温,导致甲烷在正常稳定区无法结冰。地球海洋内的沉积表面已经有破洞,称为坑洞[资料来源: Hill et al., 2004 ],说明了这些爆出的气体为什么看起来像来自表面。
另外还有发生崩移的可能性,当水合物溶解产生气泡时,体积会增大。这种说法是认为气泡能使结晶彼此脱落,导致沉积柱 ( 层 ) 不稳定。众所皆知最大的海底崩移为 Storegga( 挪威语:巨大的边缘 ) ,就在挪威附近沿海[ 资料来源: Bryn et al., 2005; Mienert et al., 2005 ]这个 崩移效应挖凿出约 250 公尺 高、超过数百公里宽的沉积层,几乎跨越挪威到格陵兰岛之间的一半距离。挪威边境大概每 10 万年, 会发生与冰河周期同步的类似地层滑动。[ 资料来源 : Solheim et al., 2005 ]
最近一次是出现在稳定区因水温增高而变薄的 2-3 千年之后[ 资料来源 : Mienert et al., 2005 ],即约在 8150 年前。地层滑动由水底数百公尺深的地方开始,刚好在陆坡边缘, Mienert 由此计算出水合物稳定带 (HSZ) 的最大变化。 Storegga 崩移区包含的甲烷气水包合物囤积量,根据地震 BSR 分析的结果,与 200-300 公尺 HSZ 的基地所在相符合,并且坑洞也指出气体自沉积处爆出的现象。
但是,也有其他对 Storegga 抱持 振振有词 的假设,认为它跟水合物一点关系也没有。该冰河沉积棚系 芬诺斯堪迪亚 ( Fennoscandian) 冰床的迅速囤积结果,[ 资料来源: Bryn et al., 2005 ] 快速沉积活动对装载于沉积柱内的孔隙水, 比因为沉积增加而排除的 孔隙水 来得快,有 些观点认为,沉积柱的浮动系仰赖自身的孔隙水。这种机制可以充分解释,为什么挪威边境的陆块,世界各地也一样,会因气候的变化,同步导致崩移的现象。
Storegga 崩移曾为现在的英国带来海啸,但未有任何迹象显示与气候有关。那时甲烷流失并未到达灾变量,沉积层移动约 2500 立方公里的体积,假设由崩移量中,平均有 1% 的水合物藉由孔隙水释放出来,即有 0.8G ton 的含碳甲烷获得释放,即便所有的水合物都释放到大气中,对气候的冲击将比火山爆发略小一点 ( 在此以辐射平衡计算甲烷的影响 ) 。
实际上,事实已不言可喻, Storegga 崩移现象,在 8.2 千年的气候异常如影随形,但并未有任何迹象显示它们之间有所关联。 8.2 千年的气候异常是长达一世纪酷冷时期,大致原因是淡水从 Aggasiz 冰湖释放到北大西洋,同时甲烷浓度下降 75ppbv ,并非上升。
甲烷可以叁种可能的形式脱离沉积层,即溶解、气泡与水合物。甲烷溶化后在好氧深层海水里,化学性质不稳定,但可以维持几十年的光景 ( 比高流体环境短一些 ) [ 资料来源: Valentine et al., 2001 ],所以如果甲烷在海洋中被释放处浅一些,就有很好的机会可以逃脱到大气中。气泡甲烷则很典型地,在溶解之前,只能上升几百公尺。水合物则在水中载浮,就像一般的浮冰一样,其将甲烷带到大气的效率,比气泡为佳[资料来源: Brewer et al., 2002 ]。
海洋中大部分水合物的溶解过程相当漫长。需耗费数十年到几世纪,才能暖化海平面下 1000 公尺 深的海水,且需要超过好几百年使热气扩散到沉积层里,也就是稳定区基地所在。北冰洋也许就是一个特例,由于水域较冷,导致稳定区较浅,而且位于高纬度,也使暖化比预期中更为强烈。
永冻土 -- 最近各位也许已在论文中读过很多有关永冻土的资讯。永冻土被定义为终年结冰的土 ( 事实上,技术定义上是过去 2 年呈结冰状态的土 ) ,有时靠近沉积层表面的区域会在夏天融化。在永冻土的文献中,该区域称为活跃区,而且据观测显示,它会随着时间增加而扩大。[资料来源 : Sazonova et al., 2004 ]。地表土坯的融化,是高纬度北极地带,之所以被预期会成为陆地对气候的改变反应最剧烈区域的理由之一[ 资料来源: Bala et al., 2005 ]
另外一个原因是,高纬度气温的改变,比起全球变动平 均值更为剧烈,特别是在高北纬度。曾有一则有关北极陆地永冻土融化的趣闻,例如在费尔班克斯市 (Fairbanks) 附近倾斜的建筑和「醉林」[资料来源: Pearce, 2005; Stockstad, 2004 ],另外还有 更多阿拉斯加的油管是固定在永冻土上。
水合物有时是与永冻土的积存有关,但并非接近地表的永冻土,因水合物需要高压环境。另一个决定因素是水合物在土壤中是否具有渗透性。有时冻结和流动的地下水,会在土中形成一个密封的冰层,因此造成下方孔隙间的压力升高。有报告指出,水合物在一处永冻土的核心里[资料来源: Dallimore and Collett, 1995 ],系位于密封冰层的下方。亦有报告指出,湖泊突然被抽干,会对地表层密封冰层,造成很明显的破坯。地表层密封冰层的始祖,是在西伯利亚,一个非常大规模的复合冰层。[资料来源: Hubberten and Romanovskii, 2001 ]
侵蚀复合冰层最重要的方式是由侧蚀旁蚀,经由热喀斯特 (thermokarst) 侵蚀过程溶解永冻土[ 资料来源: Gavrilov et al., 2003 ]。冰层暴露于海洋暖化的水中。当冰块融化,陆地崩塌,曝露更多的冰。西伯利亚北海岸已被侵蚀数千年之久,然而,侵蚀速率仍在增加当中。整个岛屿消失在历史洪流中[资料来源 : Romankevich, 1984 ]。西伯利亚大陆棚的甲烷溶解浓度,比在大气中的浓度,高出 25 倍之多,表示逸出的甲烷系由沿岸侵蚀进入大气中[资料来源: Gornitz and Fung, 1994 ]。人类对永冻土内甲烷冰的总含量,所知有限,估计从 7.5 到 400G ton C( 本估计值由[ Gornitz and Fung, 1994 ]汇编 ) 。
未来 。最精采的灾难电影模式,可能是在一段和甲烷存在周期相当的时间里,释放出大量足以改变大气浓度的甲烷,这对甲烷浓度会形成增强效果。甲烷释放的规模到何种程度堪称为大,甲烷量必须加计因辐射驱力而倍增的一氧化碳,约相当于目前甲烷浓度的十倍威力。这才叫灾难电影。或者,到 2050 年,最糟糕的联合国政府间气候变化专门委员会 (IPCC) 模式,和想像得到的最好的」替代方案」,其辐射能量作用力的差别平均值,仅约每平方米 1 瓦 (1 W/m2) 。在这种情况下,甲烷产生的辐射驱力,将导致其无法维持在低于「危险」边缘 2 度,而到达高出工业革命前的水平。在此我预估当今甲烷浓度约 6ppm 为 1 W/m2 。以 6ppm 的甲烷浓度而言,在现实世界中确实是大难临头。

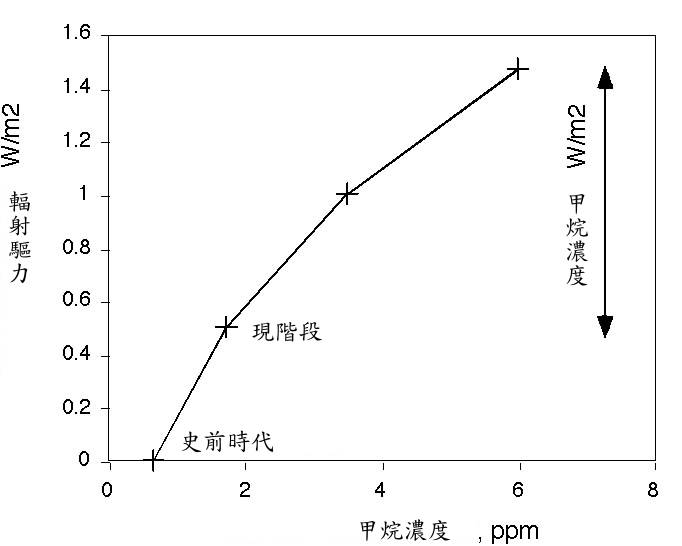
大气中一般含有约 3.5Gton C 甲烷,若在瞬间释放 10Gton C ,将会突破之前的 6ppm 浓度。这种浩劫很可能比任何被提过的灾难还要大。
海底崩移会释放约十亿吨,坑洞爆炸则略少。永冻土水合物正在溶解,但没有人想到它们会突然爆炸。根据记载,在五千五百万年前,沉积层发生所谓的古/始新世交替时期最大暖流 (Paleocene Eocene Thermal Maximum) , ( 据宣称 ) 释放几千 Gton C 甲烷到大气及海洋中,使中深层的海水升温摄氏 5 度。要推测在这么久之前的发生速度实非易事,但最佳的推测是,那些甲烷的释放期间系超过一千年之久,换言之,不是突发事件。[资料来源: Zachos et al., 2001; Schmidt and Shindell, 2003 ]
我们的未来还有另一个可能性,即甲烷会年复一年,慢慢地长时期散放到大气中。甲烷不间断地释放,即决定大气中甲烷的累积浓度。若来源倍增,浓度就会或多或少倍增。 ( 可能略多一些,因为实际上甲烷的周期增长 ) 。甲烷会氧化成二氧化碳,形成另一种可以累积千百万年的温室气体,石化燃料所产生的二氧化碳亦然。由甲烷的慢性释放模型经常可以看出,累积的二氧化碳,对暖化的贡献与甲烷不分轩轾。
人为所产生的甲烷像种稻、石化燃料产业和畜牧,使大气中的甲烷浓度,已经超过工业革命之前的水平,目前甲烷状态尚呈现稳定,但是相对于现阶段的活动,其持稳原因则尚不明确。永冻土的甲烷水合物含量尚不明朗,但不需要太多的甲烷,例如 100 年释放 60 Gton ,即可让大气中的甲烷浓度再增加两倍。沉积的泥煤,也许可以和永冻土水合物溶解产生的甲烷相比拟,当冰冻几千万年的泥煤解冻时,它仍旧含有能催化甲烷产生的甲烷氧化[资料来源: Rivkina et al., 2004 ],便开始将泥煤转变成二氧化碳和甲烷。那也就不难想像泥煤里, 60 GtonC 经过 100 年后,会是什么局面。在现存的湿地和沼泽地,甲烷的产生,受到雨量和气温的改变,产生的变化也很重要。已有预测显示,海洋水合物在溶解,但速度很缓慢[资料来源: Harvey and Huang, 1995 ]。速度较明显 的,似乎只有北极和墨西哥湾。
所以,最后的重点不是要策划一场灾难电影,而是在传达潜在的危机,对该危机的正面回馈,是否能扭转「危险」人类所致气候改变的结果,只有一线之隔。这才是可怕之处。
我已上传一份有关水合物及气候改变的详细资料,供同行审阅和刊物发表,请按此前往 。
Bala, G., K. Caldeira, A. Mirin, M. Wickett, and C. Delira, Multicentury changes to the global climate and carbon cycle: Results from a coupled climate and carbon cycle model, Journal of Climate, 18, 4531-4544, 2005.
Brewer, P.G., C. Paull, E.T. Peltzer, W. Ussler, G. Rehder, and G. Friederich, Measurements of the fate of gas hydrates during transit through the ocean water column, Geophysical Research Letters, 29 (22), 2002.
Bryn, P., K. Berg, C.F. Forsberg, A. Solheim, and T.J. Kvalstad, Explaining the Storegga Slide, Marine and Petroleum Geology, 22 (1-2), 11-19, 2005.
Buffett, B., and D.E. Archer, Global inventory of methane clathrate: Sensitivity to changes in environmental conditions, Earth and Planetary Science Letters, 227, 185-199, 2004.
Buffett, B.A., Clathrate hydrates, Annual Review of Earth and Planetary Sciences, 28, 477-507, 2000.
Dallimore, S.R., and T.S. Collett, Intrapermafrost Gas Hydrates from a Deep Core-Hole in the Mackenzie Delta, Northwest-Territories, Canada, Geology, 23 (6), 527-530, 1995.
Gavrilov, A.V., X.N. Romanovskii, V.E. Romanovsky, H.W. Hubberten, and V.E. Tumskoy, Reconstruction of ice complex remnants on the eastern Siberian Arctic Shelf, Permafrost and Periglacial Processes, 14 (2), 187-198, 2003.
Gornitz, V., and I. Fung, Potential distribution of methane hydrate in the world's oceans, Global Biogeochemical Cycles, 8, 335-347, 1994.
Harvey, L.D.D., and Z. Huang, Evaluation of the potential impact of methane clathrate destabilization on future global warming, J. Geophysical Res., 100, 2905-2926, 1995.
Hill, J.C., N.W. Driscoll, J.K. Weissel, and J.A. Goff, Large-scale elongated gas blowouts along the US Atlantic margin, Journal of Geophysical Research-Solid Earth, 109 (B9), 2004.
Hubberten, H.W., and N.N. Romanovskii, Terrestrial and offshore permafrost evolution of the Laptev sea region during the last Pleistocene-Holocene glacial-eustatic cycle, in Permafrost response on economic develoopment, environmental security and natural resources, edited by R. Paepa, and V. Melnikov, pp. 43-60, Klewer, Amsterdam, 2001.
MacDonald, I.R., L.C. Bender, M. Vardaro, B. Bernard, and J.M. Brooks, Thermal and visual time-series at a seafloor gas hydrate deposit on the Gulf of Mexico slope, Earth and Planetary Science Letters, 233 (1-2), 45-59, 2005.
Mienert, J., M. Vanneste, S. Bunz, K. Andreassen, H. Haflidason, and H.P. Sejrup, Ocean warming and gas hydrate stability on the mid-Norwegian margin at the Storegga Slide, Marine and Petroleum Geology, 22 (1-2), 233-244, 2005.
Milkov, A.V., Global estimates of hydrate-bound gas in marine sediments: how much is really out there., Earth-Science Reviews, 66 (3-4), 183-197, 2004.
Pearce, F., Climate warning as Siberia melts, New Scientist, Aug. 11, 2005.
Rivkina, E., K. Laurinavichius, J. McGrath, J. Tiedje, V. Shcherbakova, and D. Gilichinsky, Microbial life in permafrost, in Space Life Sciences: Search for Signatures of Life, and Space Flight Environmental Effects on the Nervous System, pp. 1215-1221, 2004.
Rogner, H.-H., An assessment of world hydrocarbon resources, Annu. Rev. Energy Environ., 22, 217-262, 1997.
Romankevich, E.A., Geochemistry of Organic Matter in the Ocean, Springer, New York, 1984.
Sazonova, T.S., V.E. Romanovsky, J.E. Walsh, and D.O. Sergueev, Permafrost dynamics in the 20th and 21st centuries along the East Siberian transect, Journal of Geophysical Research-Atmospheres, 109 (D1), 2004.
Shakhova, N., I. Semiletov, and G. Panteleev, The distribution of methane on the Siberian Arctic shelves: Implications for the marine methane cycle, Geophysical Research Letters, 32 (9), 2005.
Solheim, A., K. Berg, C.F. Forsberg, and P. Bryn, The Storegga Slide complex: repetitive large scale sliding with similar cause and development, Marine and Petroleum Geology, 22 (1-2), 97-107, 2005.
Schmidt, G.A., and D.T. Shindell. Atmospheric composition, radiative forcing, and climate change as a consequence of a massive methane release from gas hydrates. Paleoceanography 18, no. 1, 1004, 2003.
Stockstad, E., Defrosting the carbon freezer of the North, Science, 304, 1618-1620, 2004.
Valentine, D.L., D.C. Blanton, W.S. Reeburgh, and M. Kastner, Water column methane oxidation adjacent to an area of active hydrate dissociation, Eel River Basin, Geochimica Et Cosmochimica Acta, 65 (16), 2633-2640, 2001.
Wood, W.T., J.F. Gettrust, N.R. Chapman, G.D. Spence, and R.D. Hyndman, Decreased stability of methane hydrates in marine sediments owing to phase-boundary roughness, Nature, 420 (6916), 656-660, 2002.
Zachos, J.C., M. Pagani, L. Sloan, E. Thomas, and K. Billups, Trends, rhythms, and abberations in global climate 65 Ma to Present, Science, 292, 686-693, 2001.
source:www.realclimate.org printed with permission of author
There is an enormous amount of methane (CH
4) on earth frozen into a type of ice called methane hydrate. Hydrates can form with almost any gas and consist of a 'cage' of water molecules surrounding the gas. (The term 'clathrate' more generally describes solids consisting of gases are trapped within any kind of cage while hydrate is the specific term for when the cage is made of water molecules). There are CO
2 hydrates on Mars, while on Earth most of the hydrates are filled with methane. Most of these are in sediments of the ocean, but some are associated with permafrost soils.
Methane hydrates would seem intuitively to be the most precarious of things. Methane hydrate melts if it gets too warm, and it floats in water. Methane is a powerful
greenhouse gas, and it degrades to CO
2, another greenhouse gas which
accumulates in the atmosphere just as fossil fuel CO
2 does. And there is a lot of it, possibly more than the traditional fossil fuel deposits. Conceivably, climate changes could affect these deposits. So what do we know of the disaster-movie potential of the methane hydrates.
Ocean hydrates. Most of the methane hydrate is in sediments of the ocean. Of that, most is what can be called the stratigraphic-type deposits. Organic carbon from plankton is buried over millions of years. Hundreds of meters below the sea floor, bacteria produce methane from the dead plankton. If methane is produced quickly enough, some of it will freeze into methane hydrates. This type of deposit holds thousands of gigatons of carbon as methane [Buffett and Archer, 2004; Milkov, 2004]. For comparison, the most abundant type of traditional fossil fuel is coal, which is typically credited with about 5000 Gton C [Rogner, 1997].

Sometimes the methane moves around in the earth, and collects someplace, forming what are called structural hydrate deposits. The Gulf of Mexico, for example, is basically a leaky oil field [MacDonald et al., 2005]. One implication of gas moving around and pooling like this is that the hydrate concentration can be higher, even to the point of what they call massive deposits, lumps of nearly pure hydrate. The second bottom line is that the hydrate can be found much closer to the sea floor, and even on the sea floor.
Hydrate melts if it gets too warm. The ocean is cold enough in a depth range from say 500 meters down (200 meters in the Arctic). Below the sea floor, the temperature increases with depth, along the geothermal temperature gradient. At some depth it becomes too warm for hydrate, so hydrate melts if it becomes buried deeper than this depth. There is often a layer of bubbles beneath the hydrate stability zone. The bubbles reflect seismic sound waves, and show up clearly in seismic surveys around the world [Buffett, 2000]. Hills and valleys of the bubble layer follow hills and valleys of the sea floor, so this layer is called a bottom-simulating reflector (BSR).

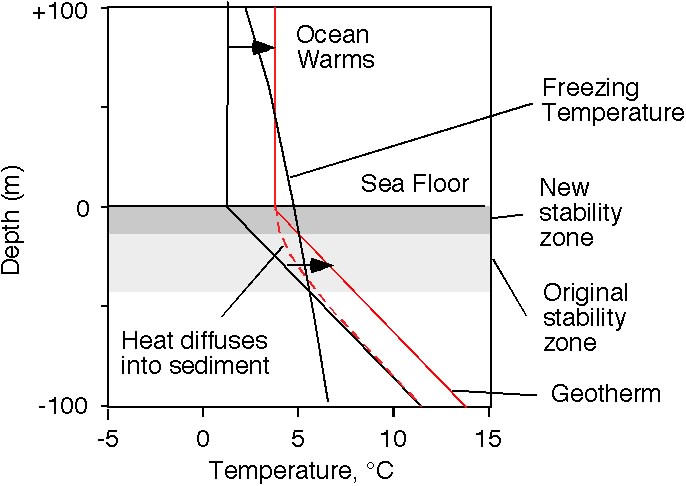
Now let's warm up the water at the top of the sediment column. Ultimately, the new temperature profile will have nearly the same slope as before, the geotherm. The hydrate stability zone will get thinner with an increase in the sediment column temperature.
The important thing to note is that it gets thinner from the bottom, not from the top. Hydrate at the base of the original stability zone finds itself melting.
If the stability zone still exists, it will be shallower in the sediment column than the newly released methane bubbles, and so it could act like a cold trap to prevent the released methane gas from escaping. However, seismic studies often show “wipeout zones” where the BSR is missing, and all of the layered structure of the sediment column above the missing BSR is smoothed out. These are thought to be areas where gas has broken through the structure of the sediment to escape to the ocean [Wood et al., 2002]. One theory is that upward migration of fluid carries with it heat, preventing the methane from freezing as it travels through the nominal stability zone. The sediment surface of the world’s ocean has holes in it called pockmarks [Hill et al., 2004], interpreted to be what these gas explosions look like from the surface.
And there is the possibility of landslides. When hydrate melts and produces bubbles, there is an increase in volume. The idea is that the bubbles might lift the grains off of each other, destabilizing the sediment column. The largest submarine landslide known is off the coast of Norway, called Storegga [Bryn et al., 2005; Mienert et al., 2005]. The slide excavated on average the top 250 meters of sediment over a swath hundreds of kilometers wide, stretching half-way from Norway to Greenland. There have been comparable slides on the Norwegian margin every approximately 100 kyr, synchronous with the glacial cycles [Solheim et al., 2005].
The last one occurred 2-3 kyr years after the stability zone thinned due to increasing water temperature [Mienert et al., 2005], about 8150 years ago. The slide started at a few hundred meters water depth, just off the continental slope, where Mienert calculates the maximum change in HSZ. The Storegga slide area today contains methane clathrate deposits as indicated by a seismic BSR corresponding to the base of the HSZ at 200-300 meters, and pockmarks indicating gas expulsion from the sediment.
However, there is another also apparently plausible hypothesis for Storegga, which doesn't involve hydrates at all. This is the rapid accumulation of glacial sediment shed by the Fennoscandian ice sheet [Bryn et al., 2005]. Rapid sediment loading traps pore water in the sediment column faster than it can be expelled by the increasing sediment load. At some point, the sediment column floats in its own porewater. This mechanism has the capacity to explain why the Norwegian continental margin, of all places in the world, should have landslides synchronous with climate change.
The Storegga slide generated a tsunami in what is now the United Kingdom, but it does not appear to have had any climate connections. It was not a catastrophic amount of methane loss. The volume of sediment moved was about 2500 km
3. Assuming 1% hydrate by pore water volume were released on average from the slide volume, you get a methane release of about 0.8 Gton of C. Even if all of the hydrate made it to the atmosphere, it would have had a smaller climate impact than a volcanic eruption (I calculated the methane impact on the radiative budget
here).
Actually, the truth be told, the Storegga slide occurred spookily close in time to the 8.2k climate event, but there doesn't appear to be any connection. The 8.2k event was a century-long cool interval, most probably in response to fresh-water release from Glacial Lake Aggasiz to the North Atlantic and was coincident with a ~75 ppbv drop in methane, not a rise.
Methane can leave the sediment in three possible forms: dissolved, bubbles, and hydrate. Dissolved methane is chemically unstable in the oxic water column of the ocean, but it has a lifetime of decades (shorter in high-flux environments) [Valentine et al., 2001], so if the methane is released shallow enough in the ocean, it has a good chance of escaping to the atmosphere. Bubbles of methane are typically only able to rise a few hundred meters before they dissolve. Hydrate floats in water just like regular ice floats in water, carrying methane to the atmosphere much more efficiently than bubbles [Brewer et al., 2002].
For most parts of the ocean, melting of hydrates is a slow process. It takes decades to centuries to warm up the water 1000 meters down in the ocean, and centuries more to diffuse that heat down into the sediment where the base of the stability zone is. The Arctic Ocean may be a special case, because of the shallower stability zone due to the colder water column, and because warming is expected to be more intense in high latitudes.
Permafrost. You've maybe read about permafrost in the paper a lot lately. Permafrost soils are defined as those which remain frozen year-round (actually, the technical definition is a soil which has been frozen for the last two years). There is sometimes a zone near the sediment surface that thaws in the summer. In the permafrost literature, this zone is called the active zone, and it has been observed to be getting larger with time [Sazonova et al., 2004]. Melting of surface soils is one reason why the high latitude Arctic is expected to be a part of the land surface that responds most dramatically to climate change [Bala et al., 2005].
The other reason is that temperature changes are more dramatic in high latitudes than the global average, especially high northern latitudes. There has been a stream of anecdotal reports of the effects of melting permafrosts on the Arctic landscape, tilted buildings and "drunken forests" near Fairbanks, for example [Pearce, 2005; Stockstad, 2004]. Much of the Alaskan oil pipeline is anchored in permafrost soils.
Hydrates are sometimes associated with permafrost deposits, but not too close to the soil surface, because of the requirement for high pressure. The other factor that determines whether you find hydrate is the permeability of the soils. Sometimes freezing, flowing groundwater creates a sealed ice layer in the soil, which can elevate the pressure in the pore space below. Hydrate in a one permafrost core [Dallimore and Collett, 1995] was reported below sealed ice layers. Lakes have been reported to suddenly drain away as some subsurface sealed ice layer is apparently breached.
The grand-daddy of subsurface sealed ice layers is a very large structure in Siberia called the ice complex [Hubberten and Romanovskii, 2001].
The most important means of eroding the ice complex is laterally, by a melt-erosion process called thermokarst erosion [Gavrilov et al., 2003]. The ice layer is exposed to the warming waters of the ocean. As the ice melts, the land collapses, exposing more ice. The northern coast of Siberia has been eroding for thousands of years, but rates are accelerating. Entire islands have disappeared in historical time [Romankevich, 1984]. Concentrations of dissolved methane on the Siberian shelf reached 25 times higher than atmospheric saturation, indicating escape of methane from coastal erosion into the atmosphere [Shakhova et al., 2005]. Total amounts of methane hydrate in permafrost soils are very poorly known, with estimates ranging from 7.5 to 400 Gton C (estimates compiled by [Gornitz and Fung, 1994]).
The Future. The juiciest disaster-movie scenario would be a release of enough methane to significantly change the atmospheric concentration, on a time scale that is fast compared with the lifetime of methane. This would generate a spike in methane concentration. For a scale of how much would be a large methane release, the amount of methane that would be required to equal the radiative forcing of doubled CO2 would be about ten times the present methane concentration. That would be disaster movie. Or, the difference between the worst case IPCC scenario and the best conceivable 'alternative scenario' by 2050 is only about 1 W/m2 mean radiative energy imbalance. A radiative forcing on that order from methane would probably make it impossible to remain below a 'dangerous' level of 2 deg above pre-industrial. I calculate
here that it would take about 6 ppm of methane to get 1 W/m2 over present-day. A methane concentration of 6 ppm would be a disaster in the real world.

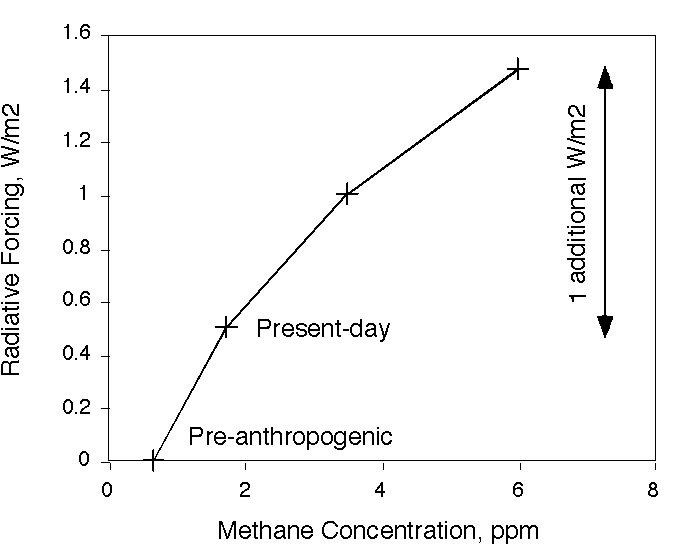
The atmosphere currently contains about 3.5 Gton C as methane. An instantaneous release of 10 Gton C would kick us up past 6 ppm. This is probably an order of magnitude larger than any of the catastrophes that anyone has proposed.
Landslides release maybe a gigaton and pockmark explosions considerably less. Permafrost hydrates are melting, but no one thinks they are going to explode all at once.
There is an event documented in sediments from 55 million years ago called the Paleocene Eocene Thermal Maximum, during which (allegedly) several thousand Gton C of methane was released to the atmosphere and ocean, driving 5° C warming of the intermediate depth ocean. It is not easy to constrain how quickly things happen so long ago, but the best guess is that the methane was released over perhaps a thousand years, i.e. not catastrophically [Zachos et al., 2001; Schmidt and Shindell, 2003].
The other possibility for our future is an increase in the year-in, year-out chronic rate of methane emission to the atmosphere. The ongoing release of methane is what supplies, and determines the concentration of, the ongoing concentration of methane in the atmosphere. Double the source, and you’d double the concentration, more or less. (A little more, actually, because the methane lifetime increases.) The methane is oxidized to CO
2, another greenhouse gas that accumulates for hundreds of thousands of years, same as fossil fuel CO
2 does. Models of chronic methane release often show that the accumulating CO
2 contributes as much to warming as does the transient methane concentration.
Anthropogenic methane sources, such as rice paddies, the fossil fuel industry, and livestock, have already more than doubled the methane concentration in the atmosphere from pre-industrial levels. Currently methane levels appear stable, but the reasons for this relatively recent phenomena are not yet clear. The amount of permafrost hydrate methane is not known very well, but it would not take too much methane, say 60 Gton C released over 100 years, to double atmospheric methane yet again. Peat deposits may be a comparable methane source to melting permafrost hydrate. When peat that has been frozen for thousands of years thaws, it still contains viable populations of methanotrophic bacteria [Rivkina et al., 2004] that begin to convert the peat into CO
2 and CH
4. It’s not too difficult to imagine 60 Gton C over 100 years from peat, either. Changes in methane production in existing wetlands and swamps due to changes in rainfall and temperature could also be important. Ocean hydrates have also been forecast to melt, but only slowly [Harvey and Huang, 1995]. Places to watch would seem to be the Arctic and the Gulf of Mexico.
So, in the end, not an obvious disaster-movie plot, but a potential positive feedback that could turn out to be the difference between success and failure in avoiding 'dangerous' anthropogenic climate change. That’s scary enough.
I have submitted a more detailed review of hydrates and climate change for peer review and publication, which can be accessed
here.
Bala, G., K. Caldeira, A. Mirin, M. Wickett, and C. Delira, Multicentury changes to the global climate and carbon cycle: Results from a coupled climate and carbon cycle model, Journal of Climate, 18, 4531-4544, 2005.
Brewer, P.G., C. Paull, E.T. Peltzer, W. Ussler, G. Rehder, and G. Friederich, Measurements of the fate of gas hydrates during transit through the ocean water column, Geophysical Research Letters, 29 (22), 2002.
Bryn, P., K. Berg, C.F. Forsberg, A. Solheim, and T.J. Kvalstad, Explaining the Storegga Slide, Marine and Petroleum Geology, 22 (1-2), 11-19, 2005.
Buffett, B., and D.E. Archer, Global inventory of methane clathrate: Sensitivity to changes in environmental conditions, Earth and Planetary Science Letters, 227, 185-199, 2004.
Buffett, B.A., Clathrate hydrates, Annual Review of Earth and Planetary Sciences, 28, 477-507, 2000.
Dallimore, S.R., and T.S. Collett, Intrapermafrost Gas Hydrates from a Deep Core-Hole in the Mackenzie Delta, Northwest-Territories, Canada, Geology, 23 (6), 527-530, 1995.
Gavrilov, A.V., X.N. Romanovskii, V.E. Romanovsky, H.W. Hubberten, and V.E. Tumskoy, Reconstruction of ice complex remnants on the eastern Siberian Arctic Shelf, Permafrost and Periglacial Processes, 14 (2), 187-198, 2003.
Gornitz, V., and I. Fung, Potential distribution of methane hydrate in the world's oceans, Global Biogeochemical Cycles, 8, 335-347, 1994.
Harvey, L.D.D., and Z. Huang, Evaluation of the potential impact of methane clathrate destabilization on future global warming, J. Geophysical Res., 100, 2905-2926, 1995.
Hill, J.C., N.W. Driscoll, J.K. Weissel, and J.A. Goff, Large-scale elongated gas blowouts along the US Atlantic margin, Journal of Geophysical Research-Solid Earth, 109 (B9), 2004.
Hubberten, H.W., and N.N. Romanovskii, Terrestrial and offshore permafrost evolution of the Laptev sea region during the last Pleistocene-Holocene glacial-eustatic cycle, in Permafrost response on economic develoopment, environmental security and natural resources, edited by R. Paepa, and V. Melnikov, pp. 43-60, Klewer, Amsterdam, 2001.
MacDonald, I.R., L.C. Bender, M. Vardaro, B. Bernard, and J.M. Brooks, Thermal and visual time-series at a seafloor gas hydrate deposit on the Gulf of Mexico slope, Earth and Planetary Science Letters, 233 (1-2), 45-59, 2005.
Mienert, J., M. Vanneste, S. Bunz, K. Andreassen, H. Haflidason, and H.P. Sejrup, Ocean warming and gas hydrate stability on the mid-Norwegian margin at the Storegga Slide, Marine and Petroleum Geology, 22 (1-2), 233-244, 2005.
Milkov, A.V., Global estimates of hydrate-bound gas in marine sediments: how much is really out there., Earth-Science Reviews, 66 (3-4), 183-197, 2004.
Pearce, F., Climate warning as Siberia melts, New Scientist, Aug. 11, 2005.
Rivkina, E., K. Laurinavichius, J. McGrath, J. Tiedje, V. Shcherbakova, and D. Gilichinsky, Microbial life in permafrost, in Space Life Sciences: Search for Signatures of Life, and Space Flight Environmental Effects on the Nervous System, pp. 1215-1221, 2004.
Rogner, H.-H., An assessment of world hydrocarbon resources, Annu. Rev. Energy Environ., 22, 217-262, 1997.
Romankevich, E.A., Geochemistry of Organic Matter in the Ocean, Springer, New York, 1984.
Sazonova, T.S., V.E. Romanovsky, J.E. Walsh, and D.O. Sergueev, Permafrost dynamics in the 20th and 21st centuries along the East Siberian transect, Journal of Geophysical Research-Atmospheres, 109 (D1), 2004.
Shakhova, N., I. Semiletov, and G. Panteleev, The distribution of methane on the Siberian Arctic shelves: Implications for the marine methane cycle, Geophysical Research Letters, 32 (9), 2005.
Solheim, A., K. Berg, C.F. Forsberg, and P. Bryn, The Storegga Slide complex: repetitive large scale sliding with similar cause and development, Marine and Petroleum Geology, 22 (1-2), 97-107, 2005.
Schmidt, G.A., and D.T. Shindell. Atmospheric composition, radiative forcing, and climate change as a consequence of a massive methane release from gas hydrates. Paleoceanography 18, no. 1, 1004, 2003.
Stockstad, E., Defrosting the carbon freezer of the North, Science, 304, 1618-1620, 2004.
Valentine, D.L., D.C. Blanton, W.S. Reeburgh, and M. Kastner, Water column methane oxidation adjacent to an area of active hydrate dissociation, Eel River Basin, Geochimica Et Cosmochimica Acta, 65 (16), 2633-2640, 2001.
Wood, W.T., J.F. Gettrust, N.R. Chapman, G.D. Spence, and R.D. Hyndman, Decreased stability of methane hydrates in marine sediments owing to phase-boundary roughness, Nature, 420 (6916), 656-660, 2002.
Zachos, J.C., M. Pagani, L. Sloan, E. Thomas, and K. Billups, Trends, rhythms, and abberations in global climate 65 Ma to Present, Science, 292, 686-693, 2001.
source:
www.realclimate.org printed with permission of author
![]()

![]()




 转寄好友
转寄好友 打印
打印












 suprememastertv.com
suprememastertv.com