甲烷冰與全球暖化
大量的甲烷 (CH 4 ) 在地球表面凍結成冰狀稱為甲烷冰,即甲烷水合物 (methane hydrate) 。該水合物幾乎可與任何氣體化合,由水分子形成籠形 (cage) 結構,將氣體團團圍住, ( 「氣水包合物 ( clathrate ) 」一詞通常是描述含有氣體的固體,被籠形結構所包圍,若該結構主要由水分子構成,即稱之為水合物 ( hydrate )) 火星上有二氧化碳,而地球充滿甲烷水合物,而多數都沉積在海床底下,但有些就成為永凍層。
甲烷冰看起來似乎是很不穩定的物質,如果天氣過於暖化,就會溶解並浮在水面上。甲烷是一種強烈的溫室氣體,它可以降解成二氧化碳,變成另一種溫室氣體,累積在大氣中,就像燃燒石化燃料產生二氧化碳一樣。而且其囤積量不容小覤,可能遠比傳統燃料生成量為多,可以想見,氣候變化會影響此類二氧化碳的囤積量,但我們對災難電影中甲烷冰的潛在危險性,了解有多少呢?
海洋水合物 -- 大部份的甲烷水合物沉澱在海裡,因此被稱為地層囤積。有機碳是來自上百萬年前的浮游生物殘骸,在數百公尺深的海床底下,細菌從死去的浮游生物製造出甲烷。假如甲烷的產生速度夠快,有一些即被凍結成甲烷冰。有數十億噸的甲烷,便以這種形式囤積在海裡﹝資料來源: Buffett and Archer, 2004; Milkov, 2004 ﹞為對照起見,碳生成量最大的傳統燃媒,其標準產碳量僅約 5000G 噸﹝資料來源 : Rogner, 1997 ﹞

有時甲烷在地底會移動並聚集在某些地方,形成所謂的結構性水合物囤積。譬如墨西哥灣,基本上是個有漏洞的油田﹝資料來源 : MacDonald et al., 2005 ﹞。 氣體的游移與聚集,意味著濃縮度會提高,甚至會高到形成塊狀礦床的程度,變成純塊狀的水合物。第二個後果是該些水合物,可以在離海底較近的地方、甚至在海床底下被找到。
氣候過於暖化會使水合物溶解。在海平面 500 公尺以下 ( 在北極為 200 公尺 ) 的海水算夠冷,在海床底下,溫度會隨深度增加而遞增,沿地溫梯度增加。若在某一深度的地底,因為太過暖化,而水合物未能埋在比原來更深的地底,就會溶解。在一個穩定的水合物區域下方,通常有一層氣泡層。氣泡所反射聲波震動訊號,可以在全球的地震勘測清楚取得﹝資料來源 : Buffett, 2000 ﹞氣泡層的丘陵與山谷,和海床的丘陵與山谷幾乎平行,所以這一層被稱為 海底仿擬反射 層 ( BSR ) 。

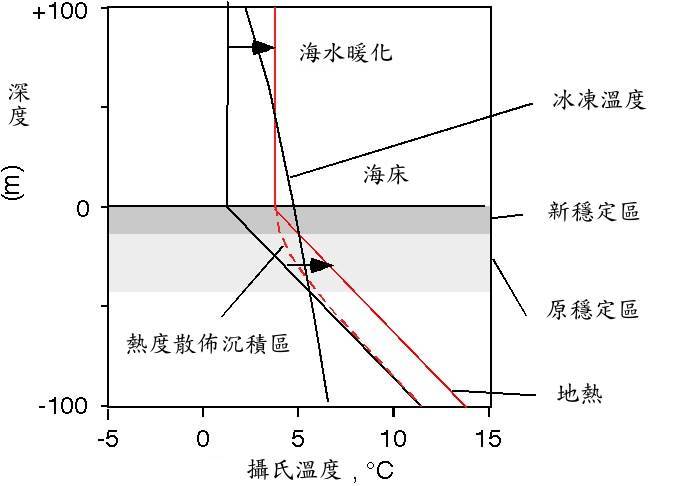
現在讓我們為 沉積柱 上方的水加溫。最後得到的地熱新溫度曲線圖,其斜率幾乎與之前的一致。水合物穩定區將因 沉積層 溫度的升高而變得更薄。
值得密切注意的是,它是從底部變薄,而不是由頂端表面變薄。水合物的原穩定區基地部分,出現自發性的溶解。
假如穩定區仍然存在,則其在沉積柱內的範圍,也要比在最新釋放出的甲烷氣泡中為淺,才能形成冷阱, 以防止甲烷氣體散失。然而在地震研究上常發現 ” 無反射區 ( wipeout zones )” ,這是海底仿擬反射的死角,且在海底仿擬反射之外的沉積層,其所有層狀結構都呈平滑狀。該些區域被認為是氣體已突破沉積結構,散發到海水裡﹝資料來源: Wood et al., 2002 ﹞有一理論主張流體往上移動時會增溫,導致甲烷在正常穩定區無法結冰。地球海洋內的沉積表面已經有破洞,稱為坑洞﹝資料來源: Hill et al., 2004 ﹞,說明了這些爆出的氣體為什麼看起來像來自表面。
另外還有發生崩移的可能性,當水合物溶解產生氣泡時,體積會增大。這種說法是認為氣泡能使結晶彼此脫落,導致沉積柱 ( 層 ) 不穩定。眾所皆知最大的海底崩移為 Storegga( 挪威語:巨大的邊緣 ) ,就在挪威附近沿海﹝ 資料來源: Bryn et al., 2005; Mienert et al., 2005 ﹞這個 崩移效應挖鑿出約 250 公尺 高、超過數百公里寬的沉積層,幾乎跨越挪威到格陵蘭島之間的一半距離。挪威邊境大概每 10 萬年, 會發生與冰河週期同步的類似地層滑動。﹝ 資料來源 : Solheim et al., 2005 ﹞
最近一次是出現在穩定區因水溫增高而變薄的 2-3 千年之後﹝ 資料來源 : Mienert et al., 2005 ﹞,即約在 8150 年前。地層滑動由水底數百公尺深的地方開始,剛好在陸坡邊緣, Mienert 由此計算出水合物穩定帶 (HSZ) 的最大變化。 Storegga 崩移區包含的甲烷氣水包合物囤積量,根據地震 BSR 分析的結果,與 200-300 公尺 HSZ 的基地所在相符合,並且坑洞也指出氣體自沉積處爆出的現象。
但是,也有其他對 Storegga 抱持 振振有詞 的假設,認為它跟水合物一點關係也沒有。該冰河沉積棚係 芬諾斯堪迪亞 ( Fennoscandian) 冰床的迅速囤積結果,﹝ 資料來源: Bryn et al., 2005 ﹞ 快速沉積活動對裝載於沉積柱內的孔隙水, 比因為沉積增加而排除的 孔隙水 來得快,有 些觀點認為,沉積柱的浮動係仰賴自身的孔隙水。這種機制可以充分解釋,為什麼挪威邊境的陸塊,世界各地也一樣,會因氣候的變化,同步導致崩移的現象。
Storegga 崩移曾為現在的英國帶來海嘯,但未有任何跡象顯示與氣候有關。那時甲烷流失並未到達災變量,沉積層移動約 2500 立方公里的體積,假設由崩移量中,平均有 1% 的水合物藉由孔隙水釋放出來,即有 0.8G ton 的含碳甲烷獲得釋放,即便所有的水合物都釋放到大氣中,對氣候的衝擊將比火山爆發略小一點 ( 在此以輻射平衡計算甲烷的影響 ) 。
實際上,事實已不言可喻, Storegga 崩移現象,在 8.2 千年的氣候異常如影隨形,但並未有任何跡象顯示它們之間有所關聯。 8.2 千年的氣候異常是長達一世紀酷冷時期,大致原因是淡水從 Aggasiz 冰湖釋放到北大西洋,同時甲烷濃度下降 75ppbv ,並非上升。
甲烷可以三種可能的形式脫離沉積層,即溶解、氣泡與水合物。甲烷溶化後在好氧深層海水裡,化學性質不穩定,但可以維持幾十年的光景 ( 比高流體環境短一些 ) ﹝ 資料來源: Valentine et al., 2001 ﹞,所以如果甲烷在海洋中被釋放處淺一些,就有很好的機會可以逃脫到大氣中。氣泡甲烷則很典型地,在溶解之前,只能上升幾百公尺。水合物則在水中載浮,就像一般的浮冰一樣,其將甲烷帶到大氣的效率,比氣泡為佳﹝資料來源: Brewer et al., 2002 ﹞。
海洋中大部分水合物的溶解過程相當漫長。需耗費數十年到幾世紀,才能暖化海平面下 1000 公尺 深的海水,且需要超過好幾百年使熱氣擴散到沉積層裡,也就是穩定區基地所在。北冰洋也許就是一個特例,由於水域較冷,導致穩定區較淺,而且位於高緯度,也使暖化比預期中更為強烈。
永凍土 -- 最近各位也許已在論文中讀過很多有關永凍土的資訊。永凍土被定義為終年結冰的土 ( 事實上,技術定義上是過去 2 年呈結冰狀態的土 ) ,有時靠近沉積層表面的區域會在夏天融化。在永凍土的文獻中,該區域稱為活躍區,而且據觀測顯示,它會隨著時間增加而擴大。﹝資料來源 : Sazonova et al., 2004 ﹞。地表土壞的融化,是高緯度北極地帶,之所以被預期會成為陸地對氣候的改變反應最劇烈區域的理由之一﹝ 資料來源: Bala et al., 2005 ﹞
另外一個原因是,高緯度氣溫的改變,比起全球變動平 均值更為劇烈,特別是在高北緯度。曾有一則有關北極陸地永凍土融化的趣聞,例如在費爾班克斯市 (Fairbanks) 附近傾斜的建築和「醉林」﹝資料來源: Pearce, 2005; Stockstad, 2004 ﹞,另外還有 更多阿拉斯加的油管是固定在永凍土上。
水合物有時是與永凍土的積存有關,但並非接近地表的永凍土,因水合物需要高壓環境。另一個決定因素是水合物在土壤中是否具有滲透性。有時凍結和流動的地下水,會在土中形成一個密封的冰層,因此造成下方孔隙間的壓力升高。有報告指出,水合物在一處永凍土的核心裡﹝資料來源: Dallimore and Collett, 1995 ﹞,係位於密封冰層的下方。亦有報告指出,湖泊突然被抽乾,會對地表層密封冰層,造成很明顯的破壞。地表層密封冰層的始祖,是在西伯利亞,一個非常大規模的複合冰層。﹝資料來源: Hubberten and Romanovskii, 2001 ﹞
侵蝕複合冰層最重要的方式是由側蝕旁蝕,經由熱喀斯特 (thermokarst) 侵蝕過程溶解永凍土﹝ 資料來源: Gavrilov et al., 2003 ﹞。冰層暴露於海洋暖化的水中。當冰塊融化,陸地崩塌,曝露更多的冰。西伯利亞北海岸已被侵蝕數千年之久,然而,侵蝕速率仍在增加當中。整個島嶼消失在歷史洪流中﹝資料來源 : Romankevich, 1984 ﹞。西伯利亞大陸棚的甲烷溶解濃度,比在大氣中的濃度,高出 25 倍之多,表示逸出的甲烷係由沿岸侵蝕進入大氣中﹝資料來源: Gornitz and Fung, 1994 ﹞。人類對永凍土內甲烷冰的總含量,所知有限,估計從 7.5 到 400G ton C( 本估計值由﹝ Gornitz and Fung, 1994 ﹞匯編 ) 。
未來 。最精采的災難電影模式,可能是在一段和甲烷存在週期相當的時間裡,釋放出大量足以改變大氣濃度的甲烷,這對甲烷濃度會形成增強效果。甲烷釋放的規模到何種程度堪稱為大,甲烷量必須加計因輻射驅力而倍增的一氧化碳,約相當於目前甲烷濃度的十倍威力。這才叫災難電影。或者,到 2050 年,最糟糕的聯合國政府間氣候變化專門委員會 (IPCC) 模式,和想像得到的最好的」替代方案」,其輻射能量作用力的差別平均值,僅約每平方米 1 瓦 (1 W/m2) 。在這種情況下,甲烷產生的輻射驅力,將導致其無法維持在低於「危險」邊緣 2 度,而到達高出工業革命前的水平。在此我預估當今甲烷濃度約 6ppm 為 1 W/m2 。以 6ppm 的甲烷濃度而言,在現實世界中確實是大難臨頭。

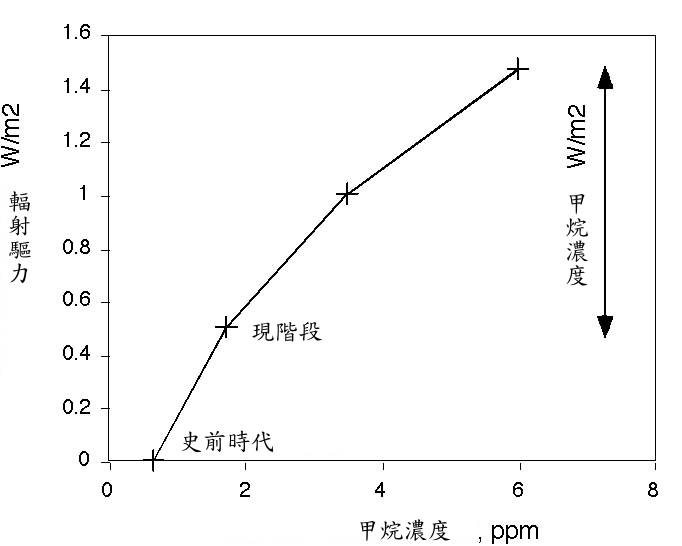
大氣中一般含有約 3.5Gton C 甲烷,若在瞬間釋放 10Gton C ,將會突破之前的 6ppm 濃度。這種浩劫很可能比任何被提過的災難還要大。
海底崩移會釋放約十億噸,坑洞爆炸則略少。永凍土水合物正在溶解,但沒有人想到它們會突然爆炸。根據記載,在五千五百萬年前,沉積層發生所謂的古/始新世交替時期最大暖流 (Paleocene Eocene Thermal Maximum) , ( 據宣稱 ) 釋放幾千 Gton C 甲烷到大氣及海洋中,使中深層的海水升溫攝氏 5 度。要推測在這麼久之前的發生速度實非易事,但最佳的推測是,那些甲烷的釋放期間係超過一千年之久,換言之,不是突發事件。﹝資料來源: Zachos et al., 2001; Schmidt and Shindell, 2003 ﹞
我們的未來還有另一個可能性,即甲烷會年復一年,慢慢地長時期散放到大氣中。甲烷不間斷地釋放,即決定大氣中甲烷的累積濃度。若來源倍增,濃度就會或多或少倍增。 ( 可能略多一些,因為實際上甲烷的周期增長 ) 。甲烷會氧化成二氧化碳,形成另一種可以累積千百萬年的溫室氣體,石化燃料所產生的二氧化碳亦然。由甲烷的慢性釋放模型經常可以看出,累積的二氧化碳,對暖化的貢獻與甲烷不分軒輊。
人為所產生的甲烷像種稻、石化燃料產業和畜牧,使大氣中的甲烷濃度,已經超過工業革命之前的水平,目前甲烷狀態尚呈現穩定,但是相對於現階段的活動,其持穩原因則尚不明確。永凍土的甲烷水合物含量尚不明朗,但不需要太多的甲烷,例如 100 年釋放 60 Gton ,即可讓大氣中的甲烷濃度再增加兩倍。沉積的泥煤,也許可以和永凍土水合物溶解產生的甲烷相比擬,當冰凍幾千萬年的泥煤解凍時,它仍舊含有能催化甲烷產生的甲烷氧化﹝資料來源: Rivkina et al., 2004 ﹞,便開始將泥煤轉變成二氧化碳和甲烷。那也就不難想像泥煤裡, 60 GtonC 經過 100 年後,會是什麼局面。在現存的濕地和沼澤地,甲烷的產生,受到雨量和氣溫的改變,產生的變化也很重要。已有預測顯示,海洋水合物在溶解,但速度很緩慢﹝資料來源: Harvey and Huang, 1995 ﹞。速度較明顯 的,似乎只有北極和墨西哥灣。
所以,最後的重點不是要策劃一場災難電影,而是在傳達潛在的危機,對該危機的正面回饋,是否能扭轉「危險」人類所致氣候改變的結果,只有一線之隔。這才是可怕之處。
我已上傳一份有關水合物及氣候改變的詳細資料,供同行審閱和刊物發表,請按此前往 。
Bala, G., K. Caldeira, A. Mirin, M. Wickett, and C. Delira, Multicentury changes to the global climate and carbon cycle: Results from a coupled climate and carbon cycle model, Journal of Climate, 18, 4531-4544, 2005.
Brewer, P.G., C. Paull, E.T. Peltzer, W. Ussler, G. Rehder, and G. Friederich, Measurements of the fate of gas hydrates during transit through the ocean water column, Geophysical Research Letters, 29 (22), 2002.
Bryn, P., K. Berg, C.F. Forsberg, A. Solheim, and T.J. Kvalstad, Explaining the Storegga Slide, Marine and Petroleum Geology, 22 (1-2), 11-19, 2005.
Buffett, B., and D.E. Archer, Global inventory of methane clathrate: Sensitivity to changes in environmental conditions, Earth and Planetary Science Letters, 227, 185-199, 2004.
Buffett, B.A., Clathrate hydrates, Annual Review of Earth and Planetary Sciences, 28, 477-507, 2000.
Dallimore, S.R., and T.S. Collett, Intrapermafrost Gas Hydrates from a Deep Core-Hole in the Mackenzie Delta, Northwest-Territories, Canada, Geology, 23 (6), 527-530, 1995.
Gavrilov, A.V., X.N. Romanovskii, V.E. Romanovsky, H.W. Hubberten, and V.E. Tumskoy, Reconstruction of ice complex remnants on the eastern Siberian Arctic Shelf, Permafrost and Periglacial Processes, 14 (2), 187-198, 2003.
Gornitz, V., and I. Fung, Potential distribution of methane hydrate in the world's oceans, Global Biogeochemical Cycles, 8, 335-347, 1994.
Harvey, L.D.D., and Z. Huang, Evaluation of the potential impact of methane clathrate destabilization on future global warming, J. Geophysical Res., 100, 2905-2926, 1995.
Hill, J.C., N.W. Driscoll, J.K. Weissel, and J.A. Goff, Large-scale elongated gas blowouts along the US Atlantic margin, Journal of Geophysical Research-Solid Earth, 109 (B9), 2004.
Hubberten, H.W., and N.N. Romanovskii, Terrestrial and offshore permafrost evolution of the Laptev sea region during the last Pleistocene-Holocene glacial-eustatic cycle, in Permafrost response on economic develoopment, environmental security and natural resources, edited by R. Paepa, and V. Melnikov, pp. 43-60, Klewer, Amsterdam, 2001.
MacDonald, I.R., L.C. Bender, M. Vardaro, B. Bernard, and J.M. Brooks, Thermal and visual time-series at a seafloor gas hydrate deposit on the Gulf of Mexico slope, Earth and Planetary Science Letters, 233 (1-2), 45-59, 2005.
Mienert, J., M. Vanneste, S. Bunz, K. Andreassen, H. Haflidason, and H.P. Sejrup, Ocean warming and gas hydrate stability on the mid-Norwegian margin at the Storegga Slide, Marine and Petroleum Geology, 22 (1-2), 233-244, 2005.
Milkov, A.V., Global estimates of hydrate-bound gas in marine sediments: how much is really out there?, Earth-Science Reviews, 66 (3-4), 183-197, 2004.
Pearce, F., Climate warning as Siberia melts, New Scientist, Aug. 11, 2005.
Rivkina, E., K. Laurinavichius, J. McGrath, J. Tiedje, V. Shcherbakova, and D. Gilichinsky, Microbial life in permafrost, in Space Life Sciences: Search for Signatures of Life, and Space Flight Environmental Effects on the Nervous System, pp. 1215-1221, 2004.
Rogner, H.-H., An assessment of world hydrocarbon resources, Annu. Rev. Energy Environ., 22, 217-262, 1997.
Romankevich, E.A., Geochemistry of Organic Matter in the Ocean, Springer, New York, 1984.
Sazonova, T.S., V.E. Romanovsky, J.E. Walsh, and D.O. Sergueev, Permafrost dynamics in the 20th and 21st centuries along the East Siberian transect, Journal of Geophysical Research-Atmospheres, 109 (D1), 2004.
Shakhova, N., I. Semiletov, and G. Panteleev, The distribution of methane on the Siberian Arctic shelves: Implications for the marine methane cycle, Geophysical Research Letters, 32 (9), 2005.
Solheim, A., K. Berg, C.F. Forsberg, and P. Bryn, The Storegga Slide complex: repetitive large scale sliding with similar cause and development, Marine and Petroleum Geology, 22 (1-2), 97-107, 2005.
Schmidt, G.A., and D.T. Shindell. Atmospheric composition, radiative forcing, and climate change as a consequence of a massive methane release from gas hydrates. Paleoceanography 18, no. 1, 1004, 2003.
Stockstad, E., Defrosting the carbon freezer of the North, Science, 304, 1618-1620, 2004.
Valentine, D.L., D.C. Blanton, W.S. Reeburgh, and M. Kastner, Water column methane oxidation adjacent to an area of active hydrate dissociation, Eel River Basin, Geochimica Et Cosmochimica Acta, 65 (16), 2633-2640, 2001.
Wood, W.T., J.F. Gettrust, N.R. Chapman, G.D. Spence, and R.D. Hyndman, Decreased stability of methane hydrates in marine sediments owing to phase-boundary roughness, Nature, 420 (6916), 656-660, 2002.
Zachos, J.C., M. Pagani, L. Sloan, E. Thomas, and K. Billups, Trends, rhythms, and abberations in global climate 65 Ma to Present, Science, 292, 686-693, 2001.
source:www.realclimate.org printed with permission of author
There is an enormous amount of methane (CH
4) on earth frozen into a type of ice called methane hydrate. Hydrates can form with almost any gas and consist of a 'cage' of water molecules surrounding the gas. (The term 'clathrate' more generally describes solids consisting of gases are trapped within any kind of cage while hydrate is the specific term for when the cage is made of water molecules). There are CO
2 hydrates on Mars, while on Earth most of the hydrates are filled with methane. Most of these are in sediments of the ocean, but some are associated with permafrost soils.
Methane hydrates would seem intuitively to be the most precarious of things. Methane hydrate melts if it gets too warm, and it floats in water. Methane is a powerful
greenhouse gas, and it degrades to CO
2, another greenhouse gas which
accumulates in the atmosphere just as fossil fuel CO
2 does. And there is a lot of it, possibly more than the traditional fossil fuel deposits. Conceivably, climate changes could affect these deposits. So what do we know of the disaster-movie potential of the methane hydrates?
Ocean hydrates. Most of the methane hydrate is in sediments of the ocean. Of that, most is what can be called the stratigraphic-type deposits. Organic carbon from plankton is buried over millions of years. Hundreds of meters below the sea floor, bacteria produce methane from the dead plankton. If methane is produced quickly enough, some of it will freeze into methane hydrates. This type of deposit holds thousands of gigatons of carbon as methane [Buffett and Archer, 2004; Milkov, 2004]. For comparison, the most abundant type of traditional fossil fuel is coal, which is typically credited with about 5000 Gton C [Rogner, 1997].

Sometimes the methane moves around in the earth, and collects someplace, forming what are called structural hydrate deposits. The Gulf of Mexico, for example, is basically a leaky oil field [MacDonald et al., 2005]. One implication of gas moving around and pooling like this is that the hydrate concentration can be higher, even to the point of what they call massive deposits, lumps of nearly pure hydrate. The second bottom line is that the hydrate can be found much closer to the sea floor, and even on the sea floor.
Hydrate melts if it gets too warm. The ocean is cold enough in a depth range from say 500 meters down (200 meters in the Arctic). Below the sea floor, the temperature increases with depth, along the geothermal temperature gradient. At some depth it becomes too warm for hydrate, so hydrate melts if it becomes buried deeper than this depth. There is often a layer of bubbles beneath the hydrate stability zone. The bubbles reflect seismic sound waves, and show up clearly in seismic surveys around the world [Buffett, 2000]. Hills and valleys of the bubble layer follow hills and valleys of the sea floor, so this layer is called a bottom-simulating reflector (BSR).

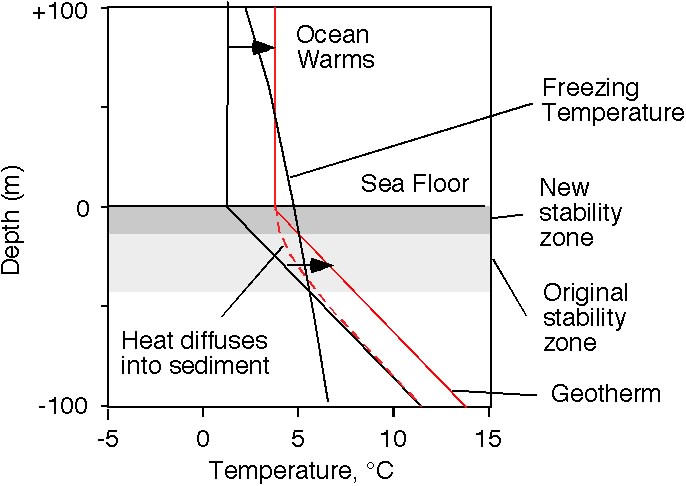
Now let's warm up the water at the top of the sediment column. Ultimately, the new temperature profile will have nearly the same slope as before, the geotherm. The hydrate stability zone will get thinner with an increase in the sediment column temperature.
The important thing to note is that it gets thinner from the bottom, not from the top. Hydrate at the base of the original stability zone finds itself melting.
If the stability zone still exists, it will be shallower in the sediment column than the newly released methane bubbles, and so it could act like a cold trap to prevent the released methane gas from escaping. However, seismic studies often show “wipeout zones” where the BSR is missing, and all of the layered structure of the sediment column above the missing BSR is smoothed out. These are thought to be areas where gas has broken through the structure of the sediment to escape to the ocean [Wood et al., 2002]. One theory is that upward migration of fluid carries with it heat, preventing the methane from freezing as it travels through the nominal stability zone. The sediment surface of the world’s ocean has holes in it called pockmarks [Hill et al., 2004], interpreted to be what these gas explosions look like from the surface.
And there is the possibility of landslides. When hydrate melts and produces bubbles, there is an increase in volume. The idea is that the bubbles might lift the grains off of each other, destabilizing the sediment column. The largest submarine landslide known is off the coast of Norway, called Storegga [Bryn et al., 2005; Mienert et al., 2005]. The slide excavated on average the top 250 meters of sediment over a swath hundreds of kilometers wide, stretching half-way from Norway to Greenland. There have been comparable slides on the Norwegian margin every approximately 100 kyr, synchronous with the glacial cycles [Solheim et al., 2005].
The last one occurred 2-3 kyr years after the stability zone thinned due to increasing water temperature [Mienert et al., 2005], about 8150 years ago. The slide started at a few hundred meters water depth, just off the continental slope, where Mienert calculates the maximum change in HSZ. The Storegga slide area today contains methane clathrate deposits as indicated by a seismic BSR corresponding to the base of the HSZ at 200-300 meters, and pockmarks indicating gas expulsion from the sediment.
However, there is another also apparently plausible hypothesis for Storegga, which doesn't involve hydrates at all. This is the rapid accumulation of glacial sediment shed by the Fennoscandian ice sheet [Bryn et al., 2005]. Rapid sediment loading traps pore water in the sediment column faster than it can be expelled by the increasing sediment load. At some point, the sediment column floats in its own porewater. This mechanism has the capacity to explain why the Norwegian continental margin, of all places in the world, should have landslides synchronous with climate change.
The Storegga slide generated a tsunami in what is now the United Kingdom, but it does not appear to have had any climate connections. It was not a catastrophic amount of methane loss. The volume of sediment moved was about 2500 km
3. Assuming 1% hydrate by pore water volume were released on average from the slide volume, you get a methane release of about 0.8 Gton of C. Even if all of the hydrate made it to the atmosphere, it would have had a smaller climate impact than a volcanic eruption (I calculated the methane impact on the radiative budget
here).
Actually, the truth be told, the Storegga slide occurred spookily close in time to the 8.2k climate event, but there doesn't appear to be any connection. The 8.2k event was a century-long cool interval, most probably in response to fresh-water release from Glacial Lake Aggasiz to the North Atlantic and was coincident with a ~75 ppbv drop in methane, not a rise.
Methane can leave the sediment in three possible forms: dissolved, bubbles, and hydrate. Dissolved methane is chemically unstable in the oxic water column of the ocean, but it has a lifetime of decades (shorter in high-flux environments) [Valentine et al., 2001], so if the methane is released shallow enough in the ocean, it has a good chance of escaping to the atmosphere. Bubbles of methane are typically only able to rise a few hundred meters before they dissolve. Hydrate floats in water just like regular ice floats in water, carrying methane to the atmosphere much more efficiently than bubbles [Brewer et al., 2002].
For most parts of the ocean, melting of hydrates is a slow process. It takes decades to centuries to warm up the water 1000 meters down in the ocean, and centuries more to diffuse that heat down into the sediment where the base of the stability zone is. The Arctic Ocean may be a special case, because of the shallower stability zone due to the colder water column, and because warming is expected to be more intense in high latitudes.
Permafrost. You've maybe read about permafrost in the paper a lot lately. Permafrost soils are defined as those which remain frozen year-round (actually, the technical definition is a soil which has been frozen for the last two years). There is sometimes a zone near the sediment surface that thaws in the summer. In the permafrost literature, this zone is called the active zone, and it has been observed to be getting larger with time [Sazonova et al., 2004]. Melting of surface soils is one reason why the high latitude Arctic is expected to be a part of the land surface that responds most dramatically to climate change [Bala et al., 2005].
The other reason is that temperature changes are more dramatic in high latitudes than the global average, especially high northern latitudes. There has been a stream of anecdotal reports of the effects of melting permafrosts on the Arctic landscape, tilted buildings and "drunken forests" near Fairbanks, for example [Pearce, 2005; Stockstad, 2004]. Much of the Alaskan oil pipeline is anchored in permafrost soils.
Hydrates are sometimes associated with permafrost deposits, but not too close to the soil surface, because of the requirement for high pressure. The other factor that determines whether you find hydrate is the permeability of the soils. Sometimes freezing, flowing groundwater creates a sealed ice layer in the soil, which can elevate the pressure in the pore space below. Hydrate in a one permafrost core [Dallimore and Collett, 1995] was reported below sealed ice layers. Lakes have been reported to suddenly drain away as some subsurface sealed ice layer is apparently breached.
The grand-daddy of subsurface sealed ice layers is a very large structure in Siberia called the ice complex [Hubberten and Romanovskii, 2001].
The most important means of eroding the ice complex is laterally, by a melt-erosion process called thermokarst erosion [Gavrilov et al., 2003]. The ice layer is exposed to the warming waters of the ocean. As the ice melts, the land collapses, exposing more ice. The northern coast of Siberia has been eroding for thousands of years, but rates are accelerating. Entire islands have disappeared in historical time [Romankevich, 1984]. Concentrations of dissolved methane on the Siberian shelf reached 25 times higher than atmospheric saturation, indicating escape of methane from coastal erosion into the atmosphere [Shakhova et al., 2005]. Total amounts of methane hydrate in permafrost soils are very poorly known, with estimates ranging from 7.5 to 400 Gton C (estimates compiled by [Gornitz and Fung, 1994]).
The Future. The juiciest disaster-movie scenario would be a release of enough methane to significantly change the atmospheric concentration, on a time scale that is fast compared with the lifetime of methane. This would generate a spike in methane concentration. For a scale of how much would be a large methane release, the amount of methane that would be required to equal the radiative forcing of doubled CO2 would be about ten times the present methane concentration. That would be disaster movie. Or, the difference between the worst case IPCC scenario and the best conceivable 'alternative scenario' by 2050 is only about 1 W/m2 mean radiative energy imbalance. A radiative forcing on that order from methane would probably make it impossible to remain below a 'dangerous' level of 2 deg above pre-industrial. I calculate
here that it would take about 6 ppm of methane to get 1 W/m2 over present-day. A methane concentration of 6 ppm would be a disaster in the real world.

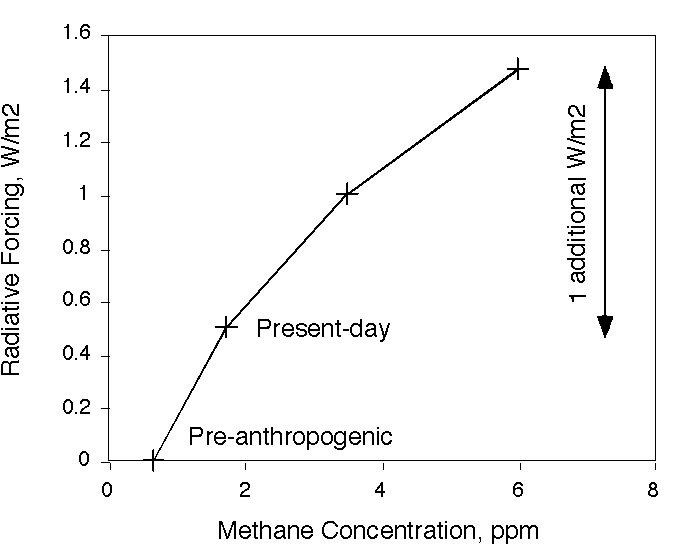
The atmosphere currently contains about 3.5 Gton C as methane. An instantaneous release of 10 Gton C would kick us up past 6 ppm. This is probably an order of magnitude larger than any of the catastrophes that anyone has proposed.
Landslides release maybe a gigaton and pockmark explosions considerably less. Permafrost hydrates are melting, but no one thinks they are going to explode all at once.
There is an event documented in sediments from 55 million years ago called the Paleocene Eocene Thermal Maximum, during which (allegedly) several thousand Gton C of methane was released to the atmosphere and ocean, driving 5° C warming of the intermediate depth ocean. It is not easy to constrain how quickly things happen so long ago, but the best guess is that the methane was released over perhaps a thousand years, i.e. not catastrophically [Zachos et al., 2001; Schmidt and Shindell, 2003].
The other possibility for our future is an increase in the year-in, year-out chronic rate of methane emission to the atmosphere. The ongoing release of methane is what supplies, and determines the concentration of, the ongoing concentration of methane in the atmosphere. Double the source, and you’d double the concentration, more or less. (A little more, actually, because the methane lifetime increases.) The methane is oxidized to CO
2, another greenhouse gas that accumulates for hundreds of thousands of years, same as fossil fuel CO
2 does. Models of chronic methane release often show that the accumulating CO
2 contributes as much to warming as does the transient methane concentration.
Anthropogenic methane sources, such as rice paddies, the fossil fuel industry, and livestock, have already more than doubled the methane concentration in the atmosphere from pre-industrial levels. Currently methane levels appear stable, but the reasons for this relatively recent phenomena are not yet clear. The amount of permafrost hydrate methane is not known very well, but it would not take too much methane, say 60 Gton C released over 100 years, to double atmospheric methane yet again. Peat deposits may be a comparable methane source to melting permafrost hydrate. When peat that has been frozen for thousands of years thaws, it still contains viable populations of methanotrophic bacteria [Rivkina et al., 2004] that begin to convert the peat into CO
2 and CH
4. It’s not too difficult to imagine 60 Gton C over 100 years from peat, either. Changes in methane production in existing wetlands and swamps due to changes in rainfall and temperature could also be important. Ocean hydrates have also been forecast to melt, but only slowly [Harvey and Huang, 1995]. Places to watch would seem to be the Arctic and the Gulf of Mexico.
So, in the end, not an obvious disaster-movie plot, but a potential positive feedback that could turn out to be the difference between success and failure in avoiding 'dangerous' anthropogenic climate change. That’s scary enough.
I have submitted a more detailed review of hydrates and climate change for peer review and publication, which can be accessed
here.
Bala, G., K. Caldeira, A. Mirin, M. Wickett, and C. Delira, Multicentury changes to the global climate and carbon cycle: Results from a coupled climate and carbon cycle model, Journal of Climate, 18, 4531-4544, 2005.
Brewer, P.G., C. Paull, E.T. Peltzer, W. Ussler, G. Rehder, and G. Friederich, Measurements of the fate of gas hydrates during transit through the ocean water column, Geophysical Research Letters, 29 (22), 2002.
Bryn, P., K. Berg, C.F. Forsberg, A. Solheim, and T.J. Kvalstad, Explaining the Storegga Slide, Marine and Petroleum Geology, 22 (1-2), 11-19, 2005.
Buffett, B., and D.E. Archer, Global inventory of methane clathrate: Sensitivity to changes in environmental conditions, Earth and Planetary Science Letters, 227, 185-199, 2004.
Buffett, B.A., Clathrate hydrates, Annual Review of Earth and Planetary Sciences, 28, 477-507, 2000.
Dallimore, S.R., and T.S. Collett, Intrapermafrost Gas Hydrates from a Deep Core-Hole in the Mackenzie Delta, Northwest-Territories, Canada, Geology, 23 (6), 527-530, 1995.
Gavrilov, A.V., X.N. Romanovskii, V.E. Romanovsky, H.W. Hubberten, and V.E. Tumskoy, Reconstruction of ice complex remnants on the eastern Siberian Arctic Shelf, Permafrost and Periglacial Processes, 14 (2), 187-198, 2003.
Gornitz, V., and I. Fung, Potential distribution of methane hydrate in the world's oceans, Global Biogeochemical Cycles, 8, 335-347, 1994.
Harvey, L.D.D., and Z. Huang, Evaluation of the potential impact of methane clathrate destabilization on future global warming, J. Geophysical Res., 100, 2905-2926, 1995.
Hill, J.C., N.W. Driscoll, J.K. Weissel, and J.A. Goff, Large-scale elongated gas blowouts along the US Atlantic margin, Journal of Geophysical Research-Solid Earth, 109 (B9), 2004.
Hubberten, H.W., and N.N. Romanovskii, Terrestrial and offshore permafrost evolution of the Laptev sea region during the last Pleistocene-Holocene glacial-eustatic cycle, in Permafrost response on economic develoopment, environmental security and natural resources, edited by R. Paepa, and V. Melnikov, pp. 43-60, Klewer, Amsterdam, 2001.
MacDonald, I.R., L.C. Bender, M. Vardaro, B. Bernard, and J.M. Brooks, Thermal and visual time-series at a seafloor gas hydrate deposit on the Gulf of Mexico slope, Earth and Planetary Science Letters, 233 (1-2), 45-59, 2005.
Mienert, J., M. Vanneste, S. Bunz, K. Andreassen, H. Haflidason, and H.P. Sejrup, Ocean warming and gas hydrate stability on the mid-Norwegian margin at the Storegga Slide, Marine and Petroleum Geology, 22 (1-2), 233-244, 2005.
Milkov, A.V., Global estimates of hydrate-bound gas in marine sediments: how much is really out there?, Earth-Science Reviews, 66 (3-4), 183-197, 2004.
Pearce, F., Climate warning as Siberia melts, New Scientist, Aug. 11, 2005.
Rivkina, E., K. Laurinavichius, J. McGrath, J. Tiedje, V. Shcherbakova, and D. Gilichinsky, Microbial life in permafrost, in Space Life Sciences: Search for Signatures of Life, and Space Flight Environmental Effects on the Nervous System, pp. 1215-1221, 2004.
Rogner, H.-H., An assessment of world hydrocarbon resources, Annu. Rev. Energy Environ., 22, 217-262, 1997.
Romankevich, E.A., Geochemistry of Organic Matter in the Ocean, Springer, New York, 1984.
Sazonova, T.S., V.E. Romanovsky, J.E. Walsh, and D.O. Sergueev, Permafrost dynamics in the 20th and 21st centuries along the East Siberian transect, Journal of Geophysical Research-Atmospheres, 109 (D1), 2004.
Shakhova, N., I. Semiletov, and G. Panteleev, The distribution of methane on the Siberian Arctic shelves: Implications for the marine methane cycle, Geophysical Research Letters, 32 (9), 2005.
Solheim, A., K. Berg, C.F. Forsberg, and P. Bryn, The Storegga Slide complex: repetitive large scale sliding with similar cause and development, Marine and Petroleum Geology, 22 (1-2), 97-107, 2005.
Schmidt, G.A., and D.T. Shindell. Atmospheric composition, radiative forcing, and climate change as a consequence of a massive methane release from gas hydrates. Paleoceanography 18, no. 1, 1004, 2003.
Stockstad, E., Defrosting the carbon freezer of the North, Science, 304, 1618-1620, 2004.
Valentine, D.L., D.C. Blanton, W.S. Reeburgh, and M. Kastner, Water column methane oxidation adjacent to an area of active hydrate dissociation, Eel River Basin, Geochimica Et Cosmochimica Acta, 65 (16), 2633-2640, 2001.
Wood, W.T., J.F. Gettrust, N.R. Chapman, G.D. Spence, and R.D. Hyndman, Decreased stability of methane hydrates in marine sediments owing to phase-boundary roughness, Nature, 420 (6916), 656-660, 2002.
Zachos, J.C., M. Pagani, L. Sloan, E. Thomas, and K. Billups, Trends, rhythms, and abberations in global climate 65 Ma to Present, Science, 292, 686-693, 2001.
source:
www.realclimate.org printed with permission of author
![]()

![]()




 轉寄好友
轉寄好友 列印
列印












 suprememastertv.com
suprememastertv.com