Băng mê-tan và hâm nóng toàn cầu
There is an enormous amount of methane (CH
4)
on earth frozen into a type of ice called methane hydrate. Hydrates can
form with almost any gas and consist of a 'cage' of water molecules
surrounding the gas. (The term 'clathrate' more generally describes
solids consisting of gases are trapped within any kind of cage while
hydrate is the specific term for when the cage is made of water
molecules). There are CO
2 hydrates on Mars, while on Earth
most of the hydrates are filled with methane. Most of these are in
sediments of the ocean, but some are associated with permafrost soils.
Methane
hydrates would seem intuitively to be the most precarious of things.
Methane hydrate melts if it gets too warm, and it floats in water.
Methane is a powerful
greenhouse gas, and it degrades to CO
2, another greenhouse gas which
accumulates in the atmosphere just as fossil fuel CO
2
does. And there is a lot of it, possibly more than the traditional
fossil fuel deposits. Conceivably, climate changes could affect these
deposits. So what do we know of the disaster-movie potential of the
methane hydrates?
Ocean hydrates.
Most of the methane hydrate is in sediments of the ocean. Of that, most
is what can be called the stratigraphic-type deposits. Organic carbon
from plankton is buried over millions of years. Hundreds of meters
below the sea floor, bacteria produce methane from the dead plankton.
If methane is produced quickly enough, some of it will freeze into
methane hydrates. This type of deposit holds thousands of gigatons of
carbon as methane [Buffett and Archer, 2004; Milkov, 2004]. For
comparison, the most abundant type of traditional fossil fuel is coal,
which is typically credited with about 5000 Gton C [Rogner, 1997].

Sometimes
the methane moves around in the earth, and collects someplace, forming
what are called structural hydrate deposits. The Gulf of Mexico, for
example, is basically a leaky oil field [MacDonald et al., 2005]. One
implication of gas moving around and pooling like this is that the
hydrate concentration can be higher, even to the point of what they
call massive deposits, lumps of nearly pure hydrate. The second bottom
line is that the hydrate can be found much closer to the sea floor, and
even on the sea floor.
Hydrate melts if it gets too warm. The
ocean is cold enough in a depth range from say 500 meters down (200
meters in the Arctic). Below the sea floor, the temperature increases
with depth, along the geothermal temperature gradient. At some depth it
becomes too warm for hydrate, so hydrate melts if it becomes buried
deeper than this depth. There is often a layer of bubbles beneath the
hydrate stability zone. The bubbles reflect seismic sound waves, and
show up clearly in seismic surveys around the world [Buffett, 2000].
Hills and valleys of the bubble layer follow hills and valleys of the
sea floor, so this layer is called a bottom-simulating reflector (BSR).

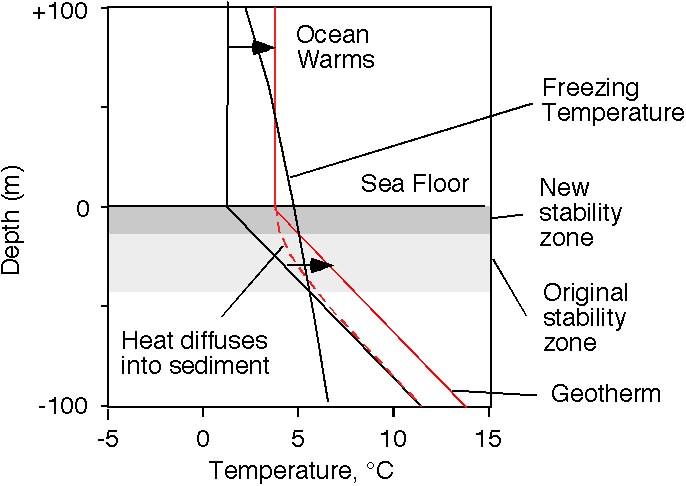
Now
let's warm up the water at the top of the sediment column. Ultimately,
the new temperature profile will have nearly the same slope as before,
the geotherm. The hydrate stability zone will get thinner with an
increase in the sediment column temperature.
The important
thing to note is that it gets thinner from the bottom, not from the
top. Hydrate at the base of the original stability zone finds itself
melting.
If the stability zone still exists, it will be
shallower in the sediment column than the newly released methane
bubbles, and so it could act like a cold trap to prevent the released
methane gas from escaping. However, seismic studies often show “wipeout
zones” where the BSR is missing, and all of the layered structure of
the sediment column above the missing BSR is smoothed out. These are
thought to be areas where gas has broken through the structure of the
sediment to escape to the ocean [Wood et al., 2002]. One theory is that
upward migration of fluid carries with it heat, preventing the methane
from freezing as it travels through the nominal stability zone. The
sediment surface of the world’s ocean has holes in it called pockmarks
[Hill et al., 2004], interpreted to be what these gas explosions look
like from the surface.
And there is the possibility of
landslides. When hydrate melts and produces bubbles, there is an
increase in volume. The idea is that the bubbles might lift the grains
off of each other, destabilizing the sediment column. The largest
submarine landslide known is off the coast of Norway, called Storegga
[Bryn et al., 2005; Mienert et al., 2005]. The slide excavated on
average the top 250 meters of sediment over a swath hundreds of
kilometers wide, stretching half-way from Norway to Greenland. There
have been comparable slides on the Norwegian margin every approximately
100 kyr, synchronous with the glacial cycles [Solheim et al., 2005].
The
last one occurred 2-3 kyr years after the stability zone thinned due to
increasing water temperature [Mienert et al., 2005], about 8150 years
ago. The slide started at a few hundred meters water depth, just off
the continental slope, where Mienert calculates the maximum change in
HSZ. The Storegga slide area today contains methane clathrate deposits
as indicated by a seismic BSR corresponding to the base of the HSZ at
200-300 meters, and pockmarks indicating gas expulsion from the
sediment.
However, there is another also apparently plausible
hypothesis for Storegga, which doesn't involve hydrates at all. This is
the rapid accumulation of glacial sediment shed by the Fennoscandian
ice sheet [Bryn et al., 2005]. Rapid sediment loading traps pore water
in the sediment column faster than it can be expelled by the increasing
sediment load. At some point, the sediment column floats in its own
porewater. This mechanism has the capacity to explain why the Norwegian
continental margin, of all places in the world, should have landslides
synchronous with climate change.
The Storegga slide generated
a tsunami in what is now the United Kingdom, but it does not appear to
have had any climate connections. It was not a catastrophic amount of
methane loss. The volume of sediment moved was about 2500 km
3.
Assuming 1% hydrate by pore water volume were released on average from
the slide volume, you get a methane release of about 0.8 Gton of C.
Even if all of the hydrate made it to the atmosphere, it would have had
a smaller climate impact than a volcanic eruption (I calculated the
methane impact on the radiative budget
here).
Actually,
the truth be told, the Storegga slide occurred spookily close in time
to the 8.2k climate event, but there doesn't appear to be any
connection. The 8.2k event was a century-long cool interval, most
probably in response to fresh-water release from Glacial Lake Aggasiz
to the North Atlantic and was coincident with a ~75 ppbv drop in
methane, not a rise.
Methane can leave the sediment in three
possible forms: dissolved, bubbles, and hydrate. Dissolved methane is
chemically unstable in the oxic water column of the ocean, but it has a
lifetime of decades (shorter in high-flux environments) [Valentine et
al., 2001], so if the methane is released shallow enough in the ocean,
it has a good chance of escaping to the atmosphere. Bubbles of methane
are typically only able to rise a few hundred meters before they
dissolve. Hydrate floats in water just like regular ice floats in
water, carrying methane to the atmosphere much more efficiently than
bubbles [Brewer et al., 2002].
For most parts of the ocean,
melting of hydrates is a slow process. It takes decades to centuries to
warm up the water 1000 meters down in the ocean, and centuries more to
diffuse that heat down into the sediment where the base of the
stability zone is. The Arctic Ocean may be a special case, because of
the shallower stability zone due to the colder water column, and
because warming is expected to be more intense in high latitudes.
Permafrost.
You've maybe read about permafrost in the paper a lot lately.
Permafrost soils are defined as those which remain frozen year-round
(actually, the technical definition is a soil which has been frozen for
the last two years). There is sometimes a zone near the sediment
surface that thaws in the summer. In the permafrost literature, this
zone is called the active zone, and it has been observed to be getting
larger with time [Sazonova et al., 2004]. Melting of surface soils is
one reason why the high latitude Arctic is expected to be a part of the
land surface that responds most dramatically to climate change [Bala et
al., 2005].
The other reason is that temperature changes are
more dramatic in high latitudes than the global average, especially
high northern latitudes. There has been a stream of anecdotal reports
of the effects of melting permafrosts on the Arctic landscape, tilted
buildings and "drunken forests" near Fairbanks, for example [Pearce,
2005; Stockstad, 2004]. Much of the Alaskan oil pipeline is anchored in
permafrost soils.
Hydrates are sometimes associated with
permafrost deposits, but not too close to the soil surface, because of
the requirement for high pressure. The other factor that determines
whether you find hydrate is the permeability of the soils. Sometimes
freezing, flowing groundwater creates a sealed ice layer in the soil,
which can elevate the pressure in the pore space below. Hydrate in a
one permafrost core [Dallimore and Collett, 1995] was reported below
sealed ice layers. Lakes have been reported to suddenly drain away as
some subsurface sealed ice layer is apparently breached.
The
grand-daddy of subsurface sealed ice layers is a very large structure
in Siberia called the ice complex [Hubberten and Romanovskii, 2001].
The
most important means of eroding the ice complex is laterally, by a
melt-erosion process called thermokarst erosion [Gavrilov et al.,
2003]. The ice layer is exposed to the warming waters of the ocean. As
the ice melts, the land collapses, exposing more ice. The northern
coast of Siberia has been eroding for thousands of years, but rates are
accelerating. Entire islands have disappeared in historical time
[Romankevich, 1984]. Concentrations of dissolved methane on the
Siberian shelf reached 25 times higher than atmospheric saturation,
indicating escape of methane from coastal erosion into the atmosphere
[Shakhova et al., 2005]. Total amounts of methane hydrate in permafrost
soils are very poorly known, with estimates ranging from 7.5 to 400
Gton C (estimates compiled by [Gornitz and Fung, 1994]).
The Future.
The juiciest disaster-movie scenario would be a release of enough
methane to significantly change the atmospheric concentration, on a
time scale that is fast compared with the lifetime of methane. This
would generate a spike in methane concentration. For a scale of how
much would be a large methane release, the amount of methane that would
be required to equal the radiative forcing of doubled CO2 would be
about ten times the present methane concentration. That would be
disaster movie. Or, the difference between the worst case IPCC scenario
and the best conceivable 'alternative scenario' by 2050 is only about 1
W/m2 mean radiative energy imbalance. A radiative forcing on that order
from methane would probably make it impossible to remain below a
'dangerous' level of 2 deg above pre-industrial. I calculate
here
that it would take about 6 ppm of methane to get 1 W/m2 over
present-day. A methane concentration of 6 ppm would be a disaster in
the real world.

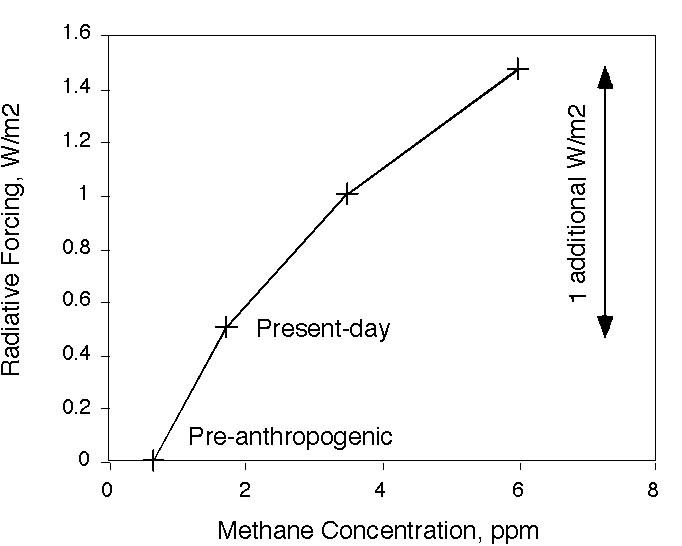
The
atmosphere currently contains about 3.5 Gton C as methane. An
instantaneous release of 10 Gton C would kick us up past 6 ppm. This is
probably an order of magnitude larger than any of the catastrophes that
anyone has proposed.
Landslides release maybe a gigaton and
pockmark explosions considerably less. Permafrost hydrates are melting,
but no one thinks they are going to explode all at once.
There is
an event documented in sediments from 55 million years ago called the
Paleocene Eocene Thermal Maximum, during which (allegedly) several
thousand Gton C of methane was released to the atmosphere and ocean,
driving 5° C warming of the intermediate depth ocean. It is not easy to
constrain how quickly things happen so long ago, but the best guess is
that the methane was released over perhaps a thousand years, i.e. not
catastrophically [Zachos et al., 2001; Schmidt and Shindell, 2003].
The
other possibility for our future is an increase in the year-in,
year-out chronic rate of methane emission to the atmosphere. The
ongoing release of methane is what supplies, and determines the
concentration of, the ongoing concentration of methane in the
atmosphere. Double the source, and you’d double the concentration, more
or less. (A little more, actually, because the methane lifetime
increases.) The methane is oxidized to CO
2, another greenhouse gas that accumulates for hundreds of thousands of years, same as fossil fuel CO
2 does. Models of chronic methane release often show that the accumulating CO
2 contributes as much to warming as does the transient methane concentration.
Anthropogenic
methane sources, such as rice paddies, the fossil fuel industry, and
livestock, have already more than doubled the methane concentration in
the atmosphere from pre-industrial levels. Currently methane levels
appear stable, but the reasons for this relatively recent phenomena are
not yet clear. The amount of permafrost hydrate methane is not known
very well, but it would not take too much methane, say 60 Gton C
released over 100 years, to double atmospheric methane yet again. Peat
deposits may be a comparable methane source to melting permafrost
hydrate. When peat that has been frozen for thousands of years thaws,
it still contains viable populations of methanotrophic bacteria
[Rivkina et al., 2004] that begin to convert the peat into CO
2 and CH
4.
It’s not too difficult to imagine 60 Gton C over 100 years from peat,
either. Changes in methane production in existing wetlands and swamps
due to changes in rainfall and temperature could also be important.
Ocean hydrates have also been forecast to melt, but only slowly [Harvey
and Huang, 1995]. Places to watch would seem to be the Arctic and the
Gulf of Mexico.
So, in the end, not an obvious
disaster-movie plot, but a potential positive feedback that could turn
out to be the difference between success and failure in avoiding
'dangerous' anthropogenic climate change. That’s scary enough.
I have submitted a more detailed review of hydrates and climate change for peer review and publication, which can be accessed
here.
Bala,
G., K. Caldeira, A. Mirin, M. Wickett, and C. Delira, Multicentury
changes to the global climate and carbon cycle: Results from a coupled
climate and carbon cycle model, Journal of Climate, 18, 4531-4544,
2005.
Brewer, P.G., C. Paull, E.T. Peltzer, W. Ussler, G. Rehder,
and G. Friederich, Measurements of the fate of gas hydrates during
transit through the ocean water column, Geophysical Research Letters,
29 (22), 2002.
Bryn, P., K. Berg, C.F. Forsberg, A. Solheim, and
T.J. Kvalstad, Explaining the Storegga Slide, Marine and Petroleum
Geology, 22 (1-2), 11-19, 2005.
Buffett, B., and D.E. Archer,
Global inventory of methane clathrate: Sensitivity to changes in
environmental conditions, Earth and Planetary Science Letters, 227,
185-199, 2004.
Buffett, B.A., Clathrate hydrates, Annual Review of Earth and Planetary Sciences, 28, 477-507, 2000.
Dallimore,
S.R., and T.S. Collett, Intrapermafrost Gas Hydrates from a Deep
Core-Hole in the Mackenzie Delta, Northwest-Territories, Canada,
Geology, 23 (6), 527-530, 1995.
Gavrilov, A.V., X.N. Romanovskii,
V.E. Romanovsky, H.W. Hubberten, and V.E. Tumskoy, Reconstruction of
ice complex remnants on the eastern Siberian Arctic Shelf, Permafrost
and Periglacial Processes, 14 (2), 187-198, 2003.
Gornitz, V., and
I. Fung, Potential distribution of methane hydrate in the world's
oceans, Global Biogeochemical Cycles, 8, 335-347, 1994.
Harvey,
L.D.D., and Z. Huang, Evaluation of the potential impact of methane
clathrate destabilization on future global warming, J. Geophysical
Res., 100,
2905-2926, 1995.
Hill,
J.C., N.W. Driscoll, J.K. Weissel, and J.A. Goff, Large-scale elongated
gas blowouts along the US Atlantic margin, Journal of Geophysical
Research-Solid Earth, 109 (B9), 2004.
Hubberten, H.W., and N.N.
Romanovskii, Terrestrial and offshore permafrost evolution of the
Laptev sea region during the last Pleistocene-Holocene glacial-eustatic
cycle, in Permafrost response on economic develoopment, environmental
security and natural resources, edited by R. Paepa, and V. Melnikov,
pp. 43-60, Klewer, Amsterdam, 2001.
MacDonald, I.R., L.C. Bender,
M. Vardaro, B. Bernard, and J.M. Brooks, Thermal and visual time-series
at a seafloor gas hydrate deposit on the Gulf of Mexico slope, Earth
and Planetary Science Letters, 233 (1-2), 45-59, 2005.
Mienert,
J., M. Vanneste, S. Bunz, K. Andreassen, H. Haflidason, and H.P.
Sejrup, Ocean warming and gas hydrate stability on the mid-Norwegian
margin at the Storegga Slide, Marine and Petroleum Geology, 22 (1-2),
233-244, 2005.
Milkov, A.V., Global estimates of hydrate-bound gas
in marine sediments: how much is really out there?, Earth-Science
Reviews, 66 (3-4), 183-197, 2004.
Pearce, F., Climate warning as Siberia melts, New Scientist, Aug. 11, 2005.
Rivkina,
E., K. Laurinavichius, J. McGrath, J. Tiedje, V. Shcherbakova, and D.
Gilichinsky, Microbial life in permafrost, in Space Life Sciences:
Search for Signatures of Life, and Space Flight Environmental Effects
on the Nervous System, pp. 1215-1221, 2004.
Rogner, H.-H., An assessment of world hydrocarbon resources, Annu. Rev. Energy Environ., 22, 217-262, 1997.
Romankevich, E.A., Geochemistry of Organic Matter in the Ocean, Springer, New York, 1984.
Sazonova,
T.S., V.E. Romanovsky, J.E. Walsh, and D.O. Sergueev, Permafrost
dynamics in the 20th and 21st centuries along the East Siberian
transect, Journal of Geophysical Research-Atmospheres, 109 (D1), 2004.
Shakhova,
N., I. Semiletov, and G. Panteleev, The distribution of methane on the
Siberian Arctic shelves: Implications for the marine methane cycle,
Geophysical Research Letters, 32 (9), 2005.
Solheim, A., K. Berg,
C.F. Forsberg, and P. Bryn, The Storegga Slide complex: repetitive
large scale sliding with similar cause and development, Marine and
Petroleum Geology, 22 (1-2), 97-107, 2005.
Schmidt, G.A., and D.T. Shindell.
Atmospheric composition, radiative forcing, and climate change as a
consequence of a massive methane release from gas hydrates.
Paleoceanography 18, no. 1, 1004, 2003.
Stockstad, E., Defrosting the carbon freezer of the North, Science, 304, 1618-1620, 2004.
Valentine,
D.L., D.C. Blanton, W.S. Reeburgh, and M. Kastner, Water column methane
oxidation adjacent to an area of active hydrate dissociation, Eel River
Basin, Geochimica Et Cosmochimica Acta, 65 (16),
2633-2640, 2001.
Wood,
W.T., J.F. Gettrust, N.R. Chapman, G.D. Spence, and R.D. Hyndman,
Decreased stability of methane hydrates in marine sediments owing to
phase-boundary roughness, Nature, 420 (6916), 656-660, 2002.
Zachos,
J.C., M. Pagani, L. Sloan, E. Thomas, and K. Billups, Trends, rhythms,
and abberations in global climate 65 Ma to Present, Science, 292,
686-693, 2001.
Nguồn:
www.realclimate.org in với sự chấp thuận của tác giả

